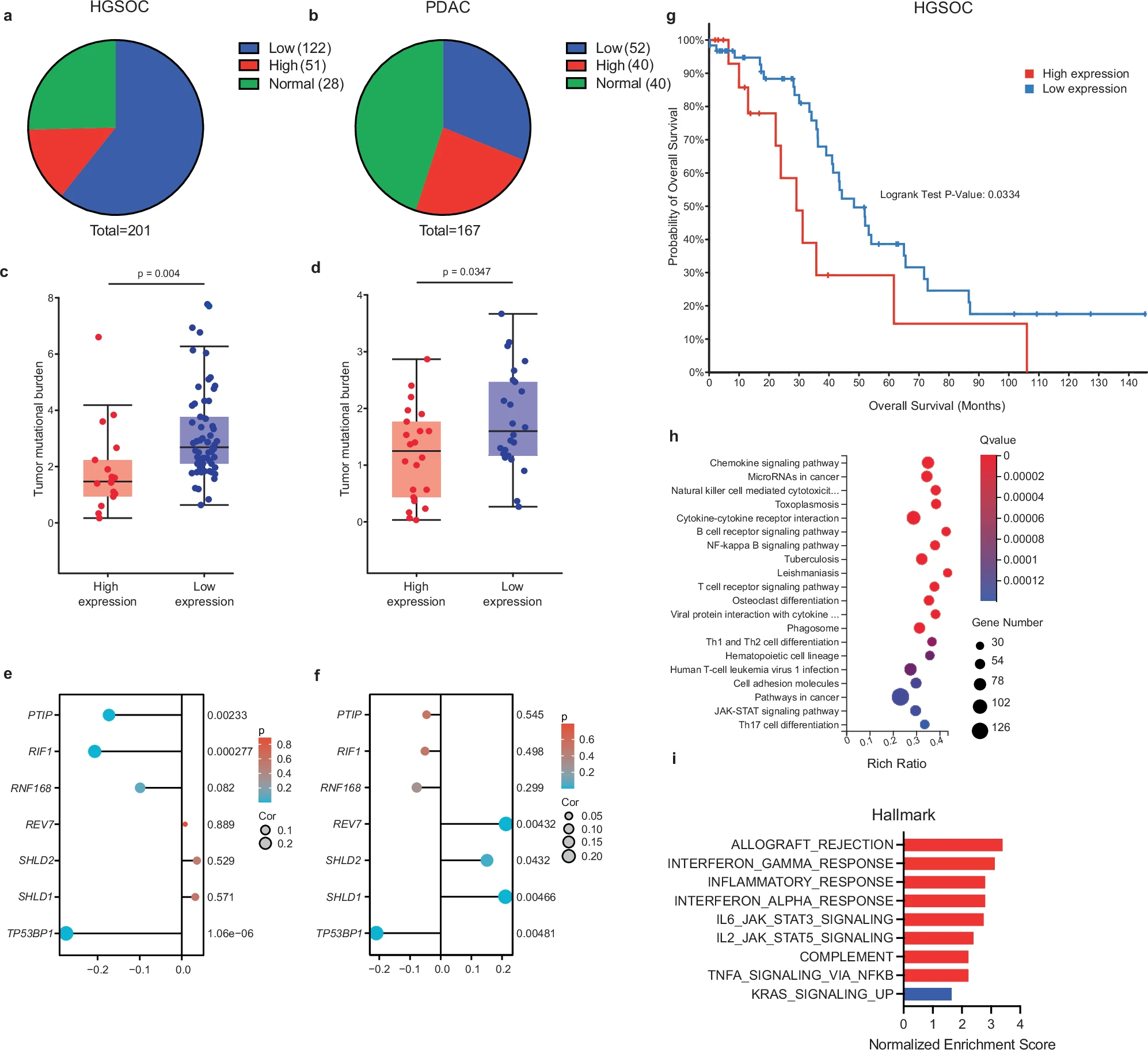
The latest article just published in the collaboration between our doctoral student Zuzanna Nowicka and Prof. Wojciech Fendler’s team with Prof. Dipanjan Chowdhury, demonstrates how loss of 53BP1, a critical regulator of DNA repair, activates the pro-inflammatory signals in cancer cells and anti-tumor immunity.
Deficiency of the molecular DNA damage repair forms the basis of modern cancer treatment methods. 53BP1 regulates the choice between two fundamental pathways for repairing double-strand DNA breaks: non-homologous end joining (NHEJ) and homologous recombination (HR). HR pathway dysfunction occurs in cells with BRCA1/2 gene mutations, making them sensitive to PARP protein inhibitors. However, a significant therapeutic challenge is posed by the resistance to PARP inhibitors, which cells with BRCA1/2 mutations can develop through the suppression of genes involved in DNA repair regulation, primarily the TP53BP1 gene. In the article, we described that the loss of 53BP1 activates the cGAS-STING pathway and pro-inflammatory signals in cancer cells. This, in turn, leads to increased tumor infiltration by cytotoxic T lymphocytes and activation of other components of anti-tumor immunity.
Although the mechanism by which factors damaging genetic material or preventing its proper repair activate an anti-tumor immune response is not new (Zuzanna Nowicka, under the supervision of Prof. Wojciech Fendler, investigates how chemoradiotherapy can alter the lung cancer immunological environment within project funded by PRELUDIUM BIS NCN grant), we have shown for the first time that loss of 53BP1 sensitizes the tumor to immunotherapy. This could potentially serve as a basis for personalized anti-cancer therapy.
You can read the full article in the open access here.
Our new publication in the Nature Communications!