We are excited to announce that Damian Mikulski, MD, PhD, has co-authored a landmark study titled “Patient-reported outcomes with belantamab mafodotin, bortezomib, and dexamethasone versus daratumumab, bortezomib, and dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-7).” This groundbreaking research has been published in the prestigious journal, The Lancet Haematology (IF: 17.7).
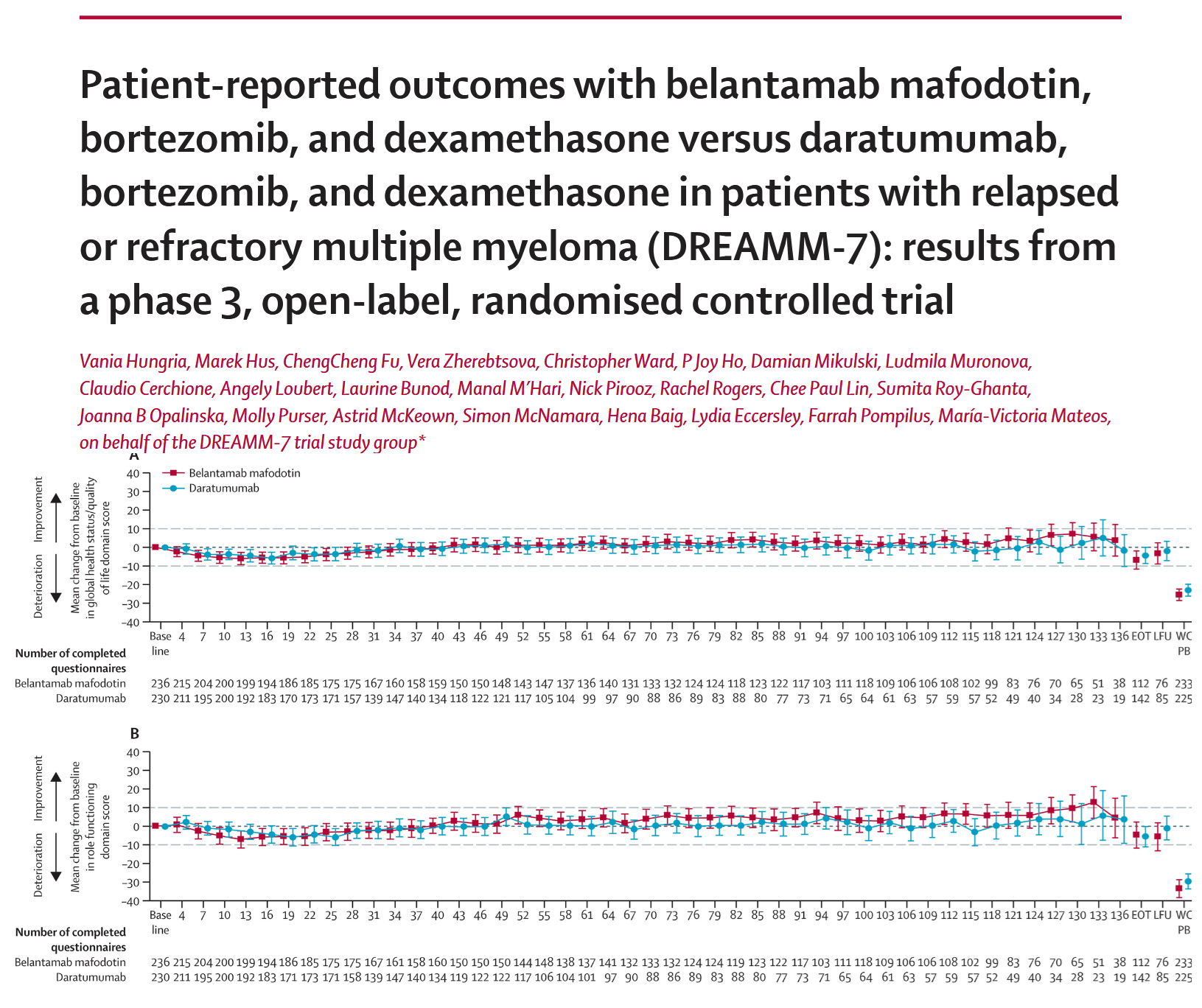
Multiple myeloma is a challenging cancer, significantly impacting patients’ quality of life. Together with an international team, Dr. Mikulski evaluated how two different treatment regimens influence health-related quality of life (HRQOL) in patients with relapsed or refractory multiple myeloma. This phase 3, open-label, randomized controlled trial was conducted across 142 hospitals in 20 countries. The study compared patient-reported outcomes between those receiving belantamab mafodotin, bortezomib, and dexamethasone and those treated with daratumumab, bortezomib, and dexamethasone. The HRQOL was generally maintained or improved over time with both treatment regimens, with manageable side effects.
This research significantly advances our understanding of multiple myeloma treatment and patient care, potentially setting new standards for therapeutic approaches!
Read the full article here.